Healthcare-associated infection (HAI) - May 10, 2022
Utilizing molecular testing to improve your SSI prevention strategy
Surgical site infections (SSIs) are one of the most common types of healthcare associated infections (HAI), with an estimated incidence of 0.5–10.1% for all surgical interventions in Europe.1 This incidence results in increased healthcare costs due to prolonged hospital and treatments, and increased mortality.2 They are the most common complication following major gastrointestinal surgery, showing in 25 – 40% of patients, with this percentage doubling from low to high income countries.3 These resulting increased healthcare costs account for £30 million a year in the UK alone.3 SSIs are post-surgery infections that occur in the part of the body where the surgery took place. They can be superficial, involving the skin only, or more serious, involving tissues under the skin or implanted material.4
Management of SSIs
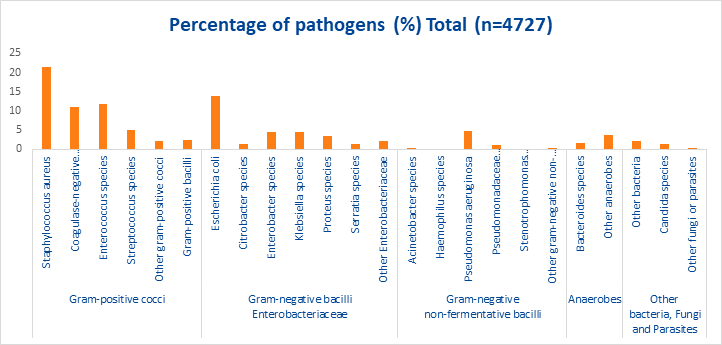
SSIs can be caused by many pathogens (see figure 1). The most common microbial cause of SSIs is Staphylococcus aureus, with studies accounting for ~21.5% of cases.5 Methicillin resistant strains (MRSA) have become the most common cause of S. aureus associated SSI.6 Adherence to proper prevention practices such as minimising operating room traffic, type of prophylactic antibiotic used, the use of hand hygiene practices, nasal screening and decolonisation of S. aureus carriers can greatly reduce the incidence of SSIs.6.
Pre-operative screening using culture or molecular-based methods followed by decolonisation of patients positive for both methicillin-susceptible S. aureus (MSSA) and MRSA reduces SSI and hospital stay.7 Nasal colonisation with S. aureus the most important independent risk factor for the development of an SSI in clean surgery.7 A randomised, double-blind, placebo-controlled, multicentre trial showed an MSSA SSI rate of 3.4% in the decolonisation treatment group compared to 7.7% in the placebo group.8
Conventional culture methods are the gold standard used to identify the causative bacteria in clinical samples.9 However, the results can take 48 hours and are dependent on the presence of viable organism.9
Figure 1. Percentages of microorganisms identified in SSIs, pooled data from 10 EU/EEA countries, 20175
Using molecular diagnostics in SSI prevention strategies
As the most common microbial cause of SSIs is Staphylococcus aureus, preoperative screening of this pathogen can greatly improve SSI prevention strategies. Rapid screening of S. aureus followed by subsequent decolonisation is shown to reduce infection rates by 60%.8 Qualitative amplification PCR that can be used to detect both MRSA and MSSA are available. In a review of one of these assays where MSSA and MRSA are distinguished, the assay showed a specificity of 99.1%.10
A recent prospective study evaluated PCR versus culture for detection of MSSA and MRSA colonisation followed by assessing any differential effects on S. aureus SSIs. Performance calculated using latent class analysis showed direct culture was the least sensitive for S. aureus (85.1%) and MRSA (76.7%), whereas the PCR assay was most sensitive for S. aureus (98.2%) and both PCR and broth-enriched culture had similar sensitivities (96.7%) for MRSA. The S. aureus-related SSI rate over 1 year was determined to be approximately 5-fold higher for MRSA culture and 10-fold for no screening compared to screening with PCR. The authors also noted that half of the S. aureus-related SSIs in this hospital population were caused by MSSA stresses the importance of screening preoperatively for both MRSA and MSSA.11
These data demonstrate that PCR screening as a step in SSI prevention strategies could enhance practices and be a better option for screening than conventional culture techniques.
References
- European Centre for Disease Prevention and Control. Healthcare-associated infections: surgical site infections. 2019. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SSI.pdf. Accessed October 2021.
- Kolasiński W. Surgical site infections – review of current knowledge, methods of prevention. Polish Journal of Surgery 2019;91:1-7.
- GlobalSurg Collaborative. Determining the worldwide epidemiology of surgical site infections after gastrointestinal resection surgery : protocol for a multicentre , international , prospective cohort study ( GlobalSurg 2 ). BMJ Open 2017;7:1-7.
- Centre for Disease Prevention and Control. Healthcare-associated Infections Surgical Site Infection ( SSI ) Resources for Patients and Healthcare Providers. Centre for Disease Control and Prevention 2021:2021. Available at: https://www.cdc.gov/hai/ssi/ssi.html. Accessed October 2021.
- European Centre for Disease Prevention and Control. Healthcare-associated infections: surgical site infections. European Centre for Disease Prevention and Control 2017. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SSI.pdf. Accessed September 9, 2021.
- Bebko SP, Green DM, Awad SS. Effect of a preoperative decontamination protocol on surgical site infections in patients undergoing elective orthopedic surgery with hardware implantation. Journal of the American Medical Association 2015;150:390-395.
- Humphreys H, Becker K, Dohmen P, et al. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. Journal of Hospital Infection 2016;94:295-304. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0195670116301657.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus aureus. New England Journal of Medicine 2010;362.
- Behera HS, Chayani N, Bal M, et al. Identification of population of bacteria from culture negative surgical site infection patients using molecular tool. BMC Surgery 2021;21:1-7.
- Mendes CE, Watters RE, Rhomberg AA, et al. Performance of BD Max StaphSR for Screening of Methicillin-Resistant Staphylococcus aureus Isolates among a Contemporary and Diverse Collection from 146 Institutions Located in Nine U.S. Census Regions: Prevalence of mecA Dropout Mutants. Journal of Clinical Microbiology 2016;54:204-207.
- Tansarli GS, LeBlanc L, Auld DB, et al. Diagnostic Accuracy of Presurgical Staphylococcus aureus PCR Assay Compared with Culture and Post-PCR Implementation Surgical Site Infection Rates. The Journal of Molecular Diagnostics 2020;22:1063-1069.